Your Neurotransmitters
Your Neurons
Your Receptors
Your Transporters
Chemicals of Interest
References
Stahl, Stephen M. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press, 2021.
PubChem, National Library of Medicine, pubchem.ncbi.nlm.nih.gov.
Disclaimer
This website does not provide medical advice. The information, including but not limited to, text, images, and other content contained, are strictly for informational purposes only. No material on this website is intended to be a substitute for professional medical advice, diagnostics, or treatment. Always seek the advice of a physician or other qualified healthcare provider with any questions you have regarding a medical condition or treatment. Never disregard professional medical advice or delay seeking medical advice because of something you have read on this website.
About
This Is Your Brain, On! is a simplified guide to your neurons and the neurotransmitters they produce.Created November 2022.
This site would not have been possible without the fantastic book "Stahl's Essential Psychopharmacology (5th edition)". You need a copy fr.Massive thanks to Stephen M. Stahl, Meghan M. Grady, and Nancy Muntner for the work put in to this indispensable resource.
Contact
Check back later.
Resources
Stahl, Stephen M. Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press, 2021.
PubChem, National Library of Medicine, pubchem.ncbi.nlm.nih.gov.
YOUR NEURONS
THE BASICS
BASICS
Your neurons communicate with each other by releasing neurotransmitters (NTs).These chemical messengers influence other neurons and sometimes other cells in your body.
Although electrical signals travel through a neuron, communication between neurons is always chemical.
NEURON ANATOMY
Neurons come in many shapes and sizes, but in general, they all have the following:
Soma: a cell body.
Dendrites: 'arms' branching off the soma which receive signals from other neurons.
Axon: the long 'tail' by which electrical signals travel away from the soma and toward other neurons like an antenna.
Axon terminal: the tip of the axon, where the electrical signal triggers chemical neurotransmitter release.
Your neurons receive chemical messages through receptors on their dendrites.This is converted to electrical signals inside the neuron. The signal travels down the axon, until it reaches the axon terminal (antenna tip), where the signal forces the neuron to release neurotransmitters and pass the message to other neurons.
Are your neurons simply passing the same message down the line? No!Each neuron makes one neurotransmitter, depending on what kind it is, but its receptors can respond to many chemicals.
NEURON FEATURES
| Receptor | alerts the neuron of what's outside when a ligand binds to it |
| Transporter | brings chemical up into neuron |
| Enzyme | a large protein (chemical chain) which can modify a smaller chemical, called the substrate. When the enzyme is done, a new product is released. |
According to StressMarq Biosciences:
There are two types of neurotransmitter receptors;
Ionotropic receptors (Ligand-gated).
Metabotropic receptors (G-protein coupled).
Ligand binding causes the opening or closing of the channel, thereby controlling the flow of ions (Na^+^, K^+^, Ca^2+^, Cl^-^) into the cell.
Examples of ionotropic receptors:
GABAA receptors
Glutamate NMDA receptors
Glutamate Kainate receptors
Glutamate AMPA receptors
Glycine receptors
Nicotinic acetylcholine (ACh) receptors (nAChR)
Serotonin 5-HT3 receptor
Metabotropic receptors do not have a channel that is not opened or closed by ligand binding. When activated they instead modulate... second messengers.
Examples of metabotropic receptors:
Adrenergic receptors
Dopamine receptors
GABAB receptors
Glutamate receptors (mGluR)
Histamine receptors
Muscarinic acetylcholine (ACh) receptors (mAChR)
Opioid receptors
Serotonin (5-HT) receptors
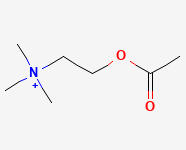
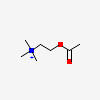
ACETYLCHOLINE SYNTHESIS
ACh synthesis begins with choline and the enzyme acetyl coenzyme A.
CHOLINE

ACETYL COENZYME A
ACETYL TRANSFER
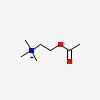
ACETYLCHOLINE
ENZYME STEPS
| Substrate | Enzyme | Effect |
|---|---|---|
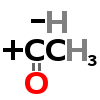
| Choline C5H14NO+ | Acetyl Coenzyme A (Acetyl-CoA) | Provides acetyl group (-COCH3) |
| ↓ | Choline Acetyltransferase (ChAT) | (-H) (+COCH3) |
| Acetylcholine C7H16NO2+ |
Why is it called acetylcholine?
There is an acetyl group (-COCH3) attached to a choline structure.
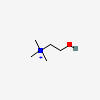
CHOLINE
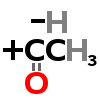
ACETYL GROUP
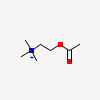
ACETYLCHOLINE
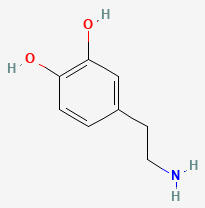
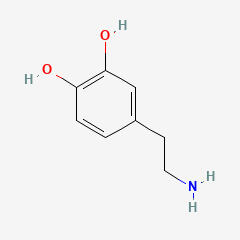
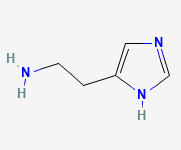
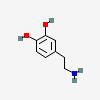
DOPAMINE (DA)
3,4-DIHYDROXYPHENETHYLAMINE
C8H11NO2
THE DOPAMINE NEURON
Dopaminergic neurons produce dopamine, which is associated with mood, reward, and motivation.
Postsynaptic (Input) Receptors
| excitatory | D1, D5 |
| inhibitory | D2, D3, D4 |
D1, D2, D3, D4, D5

D2, D3
Presynaptic Autoreceptors
| excitatory | none |
| inhibitory | D2, D3 |
TRANSPORTERS
| Dopamine transporter (DAT) | brings dopamine into neuron |
| Amino acid transporter | brings tyrosine into neuron |
OVERVIEW
| Uses | Tyrosine (Tyr) |
| Produces | Dopamine (DA) |
| Receptors | D1, D2, D3, D4, D5 |
| On DA neuron? | Yes, all DA receptors can be found on DA neurons. |
DOPAMINE SYNTHESIS
DA synthesis begins when tyrosine (TYR) is taken up into dopamine neurons.
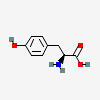
TYROSINE
HYDROXYLATION
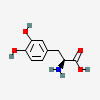
DOPA
DECARBOXYLATION
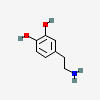
DOPAMINE
ENZYME STEPS
| Substrate | Enzyme | Effect |
|---|---|---|
| Tyrosine C9H11NO3 | ||
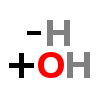
| ↓ | Tyrosine Hydroxylase (TOH) | (-H) (+OH) |
| DOPA C9H11NO4 | ||
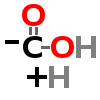
| ↓ | DOPA Decarboxylase (DDC) | (-COOH) (+H) |
| Dopamine C8H11NO2 |
Why is it called dopamine?
3,4-dihydroxyphenethylamine
There are two hydroxyl groups (dihydroxy) on the 3rd and 4th carbons (3,4) of the phenethylamine.
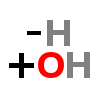
HYDROXYL
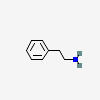
PHENETHYLAMINE
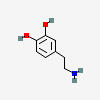
3,4-DIHYDROXYPHENETHYLAMINE
DOPAMINE RECEPTORS & TRANSPORTERS
| Name | Type | Purpose | On DA neuron? | On other neurons? |
|---|---|---|---|---|
| D1 | Receptor | Excitatory | yes | |
| D2 | Receptor | Inhibitory | yes | |
| D3 | Receptor | Inhibitory | yes | |
| D4 | Receptor | Inhibitory | yes | |
| D5 | Receptor | Excitatory | yes | |
| Tyrosine Transporter | Transporter | Brings tyrosine into DA neuron | yes | no |
| DAT | Transporter | Brings dopamine into neuron | yes | no |
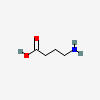
GABA is the main inhibitory neurotransmitter of the central nervous system (CNS).
GABA SYNTHESIS
GABA synthesis begins with glutamine.
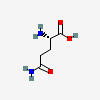
GLUTAMINE
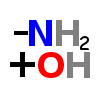
GLUTAMINASE (enzyme)
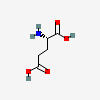
GLUTAMATE
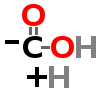
DECARBOXYLATION
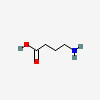
GABA
ENZYME STEPS
| Substrate | Enzyme | Effect |
|---|---|---|
| Glutamine C5H10N2O3 | ||
| ↓ | Glutaminase | (-NH2) (+OH) |
| Glutamate (glutamic acid) C5H9NO4 | ||
| ↓ | Glutamate Decarboxylase (GAD) | (-COOH) (+H) |
| GABA C4H9NO2 |
Why is it called γ-aminobutyric acid and 4-aminobutyric acid?
There is an amino group (NH2) on the fourth carbon of the butyric acid structure.
Gamma (γ) means three in chemistry. Gamma-amino structures are defined as having three carbons between the carbonyl (C=O) and amino (NH2) ends.
BUTYRIC ACID
AMINO GROUP
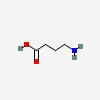
GABA
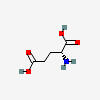
Glutamate is the main excitatory neurotransmitter of the central nervous system (CNS).
GLUTAMATE SYNTHESIS
GLU (glutamic acid) synthesis begins with glutamine.
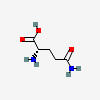
GLUTAMINE
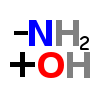
GLUTAMINASE (enzyme)
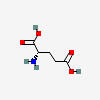
GLUTAMATE
ENZYME STEPS
| Substrate | Enzyme | Effect |
|---|---|---|
| Glutamine C5H10N2O3 | ||
| ↓ | Glutaminase | (-NH2) (+OH) |
| Glutamate (glutamic acid) C5H9NO4 |
Why is it called glutamate?
Idfk.
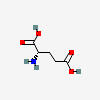
GLUTAMATE (glutamic acid)
THE HISTAMINE NEURON
Histamine neurons produce histamine, which is associated with wakefulness.
Postsynaptic (Input) Receptors
| excitatory | H1, H2 |
| inhibitory | none |
H1, H2

H3
Presynaptic Autoreceptors
| excitatory | none |
| inhibitory | H3 |
TRANSPORTERS
| Histidine transporter | brings histidine into neuron |
OVERVIEW
| Uses | Histidine |
| Produces | Histamine (HA) |
| Receptors | H1, H2, H3 |
| On HA neuron? | Yes, all HA receptors can be found on HA neurons. |
HISTAMINE SYNTHESIS
HA synthesis begins with histidine.
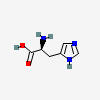
HISTIDINE
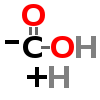
DECARBOXYLATION
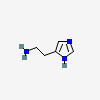
HISTAMINE
ENZYME STEPS
| Substrate | Enzyme | Effect |
|---|---|---|
| Histidine C6H9N3O2 | ||
| ↓ | Histidine Decarboxylase (HDC) | (-COOH) (+H) |
| Histamine C5H9N3 |
Why is it called histamine?
From Histamine on Wikipedia:
"By 1913 the name histamine was in use, using forms of *histo-* (tissue) and *amine* (nitrogen-group containing), yielding 'tissue amine'."
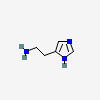
HISTAMINE
HISTAMINE RECEPTORS & TRANSPORTERS
| Name | Type | Purpose | On HA neuron? | On other neurons? |
|---|---|---|---|---|
| H1 | Postsyn. | Excitatory | yes, postsyn. | |
| H2 | Postsyn. | Excitatory | yes, postsyn. | |
| H3 | Presyn. autorecep. | Inhibitory | yes, presyn. | |
| H4 | Receptor | Not known to occur in the brain | yes | |
| Histidine Transporter | Transporter | Transports histidine into neuron | yes | |
| Histamine Transporter? | ? | Not known to exist! | n/a | n/a |
NOREPINEPHRINE SYNTHESIS
NE synthesis begins when tyrosine (TYR) is taken up into noradrenergic (norepinephrine-producing) neurons.
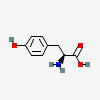
TYROSINE
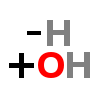
HYDROXYLATION
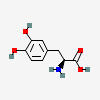
DOPA
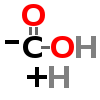
DECARBOXYLATION
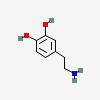
DOPAMINE
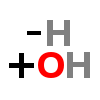
HYDROXYLATION
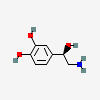
NOREPINEPHRINE
ENZYME STEPS
| Substrate | Enzyme | Effect |
|---|---|---|
| Tyrosine C9H11NO3 | ||
| ↓ | Tyrosine Hydroxylase (TOH) | (-H) (+OH) |
| DOPA C9H11NO4 | ||
| ↓ | DOPA Decarboxylase (DDC) | (-COOH) (+H) |
| Dopamine C8H11NO2 | ||
| ↓ | Dopamine β-hydroxylase (DBH) | (-H) (+OH) |
| Norepinephrine C8H11NO3 |
Why is it called norepinephrine / noradrenaline?
Norepinephrine and noradrenaline are two words for the same thing.Epinephrine is from Greek: epi- (upon) nephros (kidney). Adrenaline is named after the adrenal glands (located upon your kidneys).Nor- is an English prefix for compounds derived by removal of some part of another compound. In this case, norepinephrine is demethylated (-CH3 , +H) epinephrine/adrenaline.
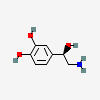
NOREPINEPHRINE (noradrenaline)
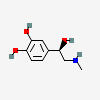
EPINEPHRINE (adrenaline)
Notice the norepinephrine molecule has one amino group, a nitrogen holding on to two hydrogens (-NH2), while epinephrine's nitrogen has one hydrogen and one methyl group (-CH3).
This site, and many others, will primarily use the term norepinephrine for the neurotransmitter itself and noradrenergic to refer to the sites of its production and effects.
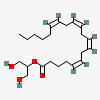
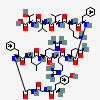
β-ENDORPHIN
C158H251N39O46S
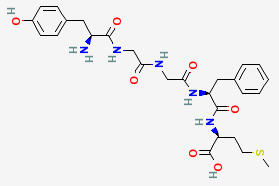
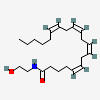
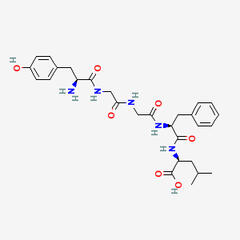
MET-ENKEPHALIN
C27H35N5O7S
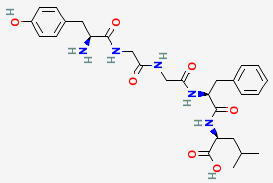
LEU-ENKEPHALIN
C28H37N5O7
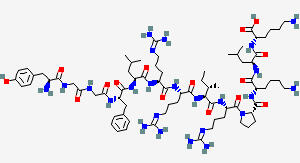
OREXIN A
C152H243N47O44S4
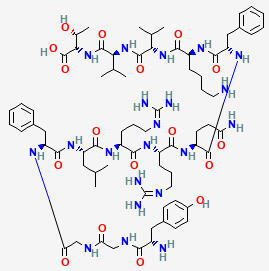
OREXIN B
C123H212N44O35S
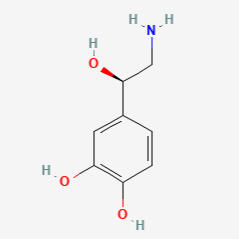
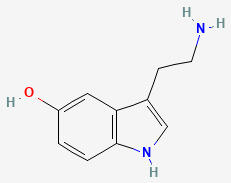
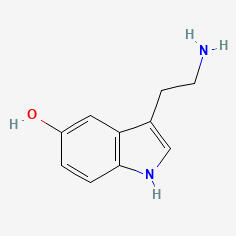
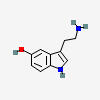
SEROTONIN (5HT)
5-HYDROXYTRYPTAMINE
C10H12N2O
SEROTONIN SYNTHESIS
5HT synthesis begins when tryptophan is taken up into serotonergic neurons.
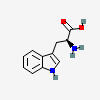
TRYPTOPHAN
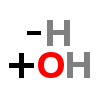
HYDROXYLATION
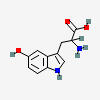
5-HYDROXYTRYPTOPHAN
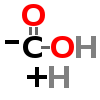
DECARBOXYLATION
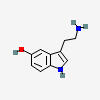
5-HYDROXYTRYPTAMINE (serotonin)
ENZYME STEPS
| Substrate | Enzyme | Effect |
|---|---|---|
| Tryptophan C11H12N2O2 | ||
| ↓ | Tryptophan Hydroxylase (TPH) | (-H) (+OH) |
| 5-hydroxytryptophan C11H12N2O3 | ||
| ↓ | Aromatic Amino Acid Decarboxylase (AADC) | (-COOH) (+H) |
| 5-hydroxytryptamine (serotonin) C10H12N2O |
Why is it called serotonin/5HT?
First extracted from intestinal cells and named enteramine by Vittorio Erspamer in 1935, serotonin was later found in blood serum in 1948. In 1952 they were found to be the same thing: 5-hydroxytryptamine (5HT, 5-HT).
Serotonin comes from the Latin serum (fluid) and tonic (medicine).
5-Hydroxytryptamine
There is a hydroxyl group on the 5th carbon of the tryptamine.
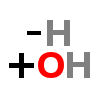
HYDROXYL
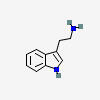
TRYPTAMINE
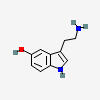
5-HYDROXYTRYPTAMINE
Animated anandamide (ANA) molecule by Mplanine.
OVERVIEW
OF YOUR NEUROTRANSMITTERS
| Name | Type | Associated with | Receptors | Transporters |
|---|---|---|---|---|
| 2-Arachidonoylglycerol (2-AG) | Endocannabinoid | Reward, "runner's high" | ||
| Acetylcholine (ACh) | Monoamine (catecholamine) | Muscle contraction, motivation, reward | Muscarinic, nicotinic | |
| Anandamide (ANA) | Endocannabinoid | Reward, "runner's high" | ||
| Dopamine (DA) | Monoamine (catecholamine) | Reward, motivation | D1, D2, D3, D4, D5 | Dopamine transporter (DAT) |
| Dynorphins | Neuropeptides | x | κ-opioid receptors | |
| Endorphins | Neuropeptides | Analgesia (pain relief) | μ-opioid receptors | |
| Enkephalins | Neuropeptides | x | δ-opioid receptors | |
| GABA | Amino acid | Signal inhibition, sleep | GABA receptors? | |
| Glutamate (GLU) | x | Signal excitation, memory, learning, wakefulness | Glutamate receptors? | |
| Glycine | Amino acid | x | x | |
| Histamine (HA) | x | Wakefulness | H1, H2, H3 | No histamine transporter has been found |
| Norepinephrine (NE) | Monoamine (catecholamine) | Arousal, wakefulness, fight/flight, habits | idk | Norepinephrine transporter (NET) |
| Orexin A | Neuropeptide | Wakefulness | OX1 | idk |
| Orexin B | Neuropeptide | Wakefulness | OX2 | idk |
| Serotonin (5HT) | Monoamine (indolamine) | Mood, motivation, digestion, vasoconstriction, vomiting | 5HT1A | Serotonin transporter (SERT) |